OTCA18 Final Project Plan – Regional hyperthermia for high-risk soft tissue sarcoma treatment – now available

The Final OTCA18 Project Plan on ‘Regional hyperthermia for high-risk soft tissue sarcoma treatment’ is now available for access, together with comments from external experts, Haukeland Hospital, and manufacturers, and the relevant answers for each from the assessment team. Please access the Final Project Plan and related documentation via the following links: OTCA18 – Final […]
Open Call for Patient Input – Joint Assessment on a medicinal product for secondary progressive MS.

EUnetHTA recently started a new Joint Assessment on a medicinal product for secondary progressive MS. To find out about participation, please read more here. Input submissions will be received through till EOB, April 28th.
PTJA04 – “Sotagliflozin is indicated as an adjunct to insulin therapy to improve glycaemic control in adults with type 1 diabetes mellitus with a Body Mass Index (BMI) ≥ 27 kg/m2, who have failed to achieve adequate glycaemic control despite optimal insulin therapy” is now available

This is the assessment of the relative effectiveness of ‘Sotagliflozin is indicated as an adjunct to insulin therapy to improve glycaemic control in adults with type 1 diabetes mellitus with a Body Mass Index (BMI) ≥ 27 kg/m2, who have failed to achieve adequate glycaemic control despite optimal insulin therapy.’ Below is the documentation provided […]
Open Call for Patient Input – Joint Assessment on a Medicinal Product for UC.
EUnetHTA recently started a new Joint Assessment on a medicinal product for the treatment of adult patients with moderately to severely active ulcerative colitis (UC), who have had an inadequate response with. lost response to, or were intolerant to either conventional therapy or a biologic, or have medical contraindications to such therapies. To find out […]
Open Call for Patient Input – Joint Assessment on a Medicinal Product for DBLCL.

EUnetHTA recently started a new Joint Assessment on a medicinal product for the treatment of patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who are not candidates for hematopoietic stem cell transplant. To find out more about how patient input can contribute to this assessment, please read further here. Input is greatly appreciated […]
OCTA20 “Prophylactic or therapeutic use of endoanchoring systems in endovascular aortic aneurysm repair (EVAR/TEVAR)” final project plan now available.
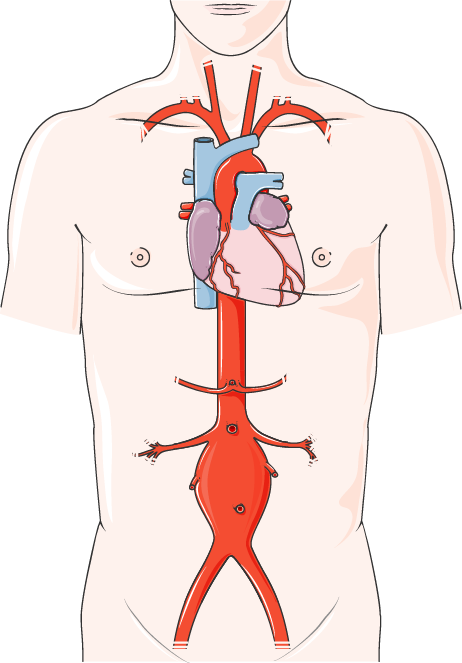
The final project plan of the assessment OTCA20 on “Prophylactic or therapeutic use of endoanchoring systems in endovascular aortic aneurysm repair (EVAR/TEVAR)” and the comments provided by external experts and the manufacturer are now available. OTCA20 Final Project Plan HERE OTCA20 External Expert & Manufacturer Comments HERE
The Collaborative Assessment, OTCA06, on “Transcatheter aortic valve implantation (TAVI) in patients at intermediate surgical risk” is now available
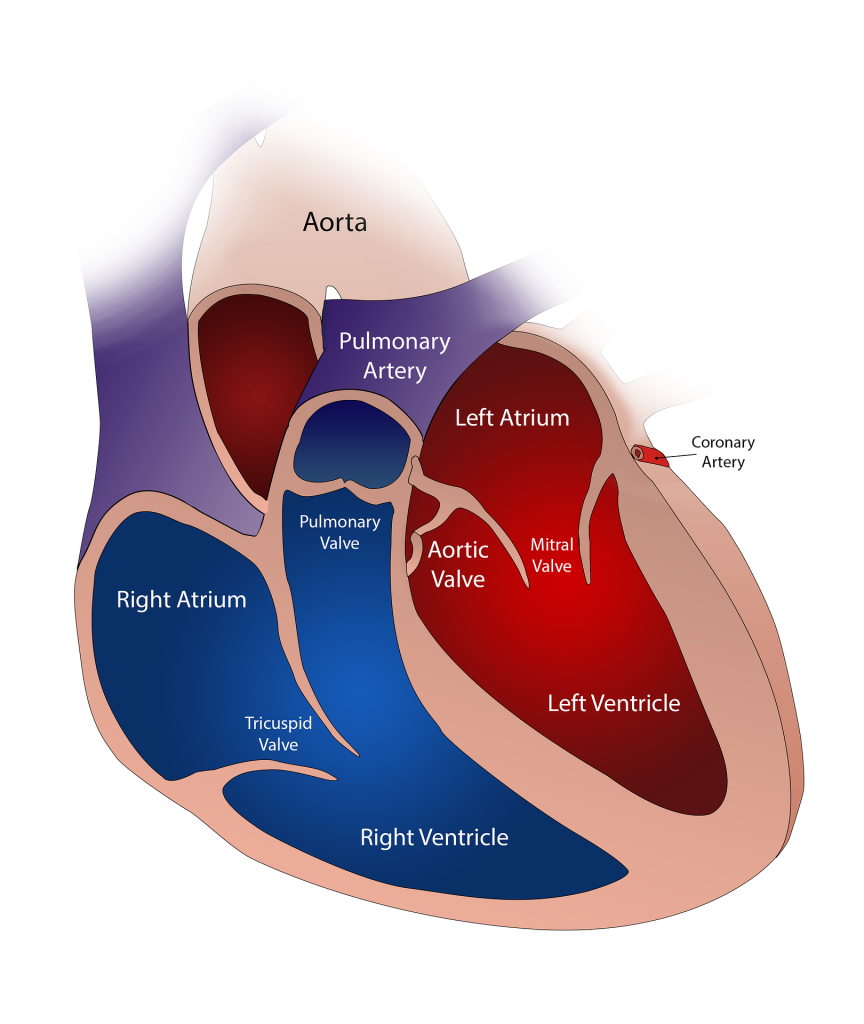
We are pleased to announce that the collaborative rapid assessment, OTCA06, on “TRANSCATHETER AORTIC VALVE IMPLANTATION (TAVI) FOR THE TREATMENT OF PATIENTS AT INTERMEDIATE SURGICAL RISK” is available. The health technology assessed is a bioprothesis in the aortic valve deployed using a catheter. The objective of this assessment was to evaluate the relative effectiveness and […]
OTCA19 “Screening for osteoporosis in the general population” project plan now available
The final project plan of the assessment OTCA19 on “Screening for osteoporosis in the general population” and the comments provided by external experts are now available. OTCA19 Project Plan HERE OTCA19 External Expert Comments HERE
Public consultation on medicinal product for relapsed or refractory Acute Myeloid Leukaemia (AML)

OTCA16 Bioresorbable Stents in cardiovascular indications (coronary artery disease) Project Plan Now Available

The final project plan of the assessment on “Bioresorbable Stents in cardiovascular indications (coronary artery disease)” and the comments provided by the external experts and the manufacturers are now available. OTCA16 Project Plan HERE OTCA16 External Expert and Manufacturer Comments HERE

