1st Collaborative Assessment – Wearable cardioverter-defibrillator (WCD) therapy in primary and secondary prevention of sudden cardiac arrest in patients at risk
Sudden cardiac arrest (SCA) is the most common cause of death in patients with coronary artery disease. Mostly, ventricular tachycardia and ventricular fibrillation are the underlying aetiology of SCA, which is claimed to be successfully treated by a novel defibrillation therapy, a wearable cardioverter defibrillator (WCD). The assessment aimed to provide valid data on clinical […]
PTJA08 – Siponimod for the treatment of adult patients with secondary progressive multiple sclerosis (SPMS) with active disease evidenced by relapses or imaging features of inflammatory activity
This is the pharmaceutical Joint Assessment PTJA08 – on siponimod for the treatment of active SPMS. In January 2020, the European Commission granted marketing authorisation for Mayzent® (siponimod) for the treatment of adult patients with secondary progressive multiple sclerosis (SPMS) with active disease evidenced by relapses or imaging features of inflammatory activity. This Joint Assessment […]
PTJA06 – Polatuzumab vedotin in combination with bendamustine and rituximab for the treatment of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who are not candidates for haematopoietic stem cell transplant
This is the pharmaceutical Joint Assessment PTJA06 – on polatuzumab vedotin for the treatment of relapsed/refractory DLBCL. In January 2020, The European Commission granted conditional marketing authorisation for Polivy® (polatuzumab vedotin), in combination with bendamustine plus MabThera® (rituximab) (BR), for the treatment of adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) […]
PTJA09 – ‘Brolucizumab for the treatment of adults with neovascular (wet) age-related macular degeneration (AMD)’ project plan now available

The final project plan of the relative effectiveness of ‘Brolucizumab for the treatment of adults with neovascular (wet) age-related macular degeneration (AMD)’ is now available for access. The final assessment report will be published on 12th March, 2020. Below is the documentation provided by the Joint Assessment authoring team. PTJA09 Final Project Plan
OTCA22 – Final Assessment Report and related documents now available

The final assessment report and related documents for the Other Technologies Collaborative Assessment ‘Point-of-care Tests (POCT): D-Dimer and Troponin’ are now available for access. Troponin and D-dimer point of care tests can be used to aid the diagnosis of patients with symptoms suggestive of suspected acute coronary syndrome and venous thromboembolism respectively. This report aimed […]
PTJA06 – “Polatuzumab vedotin in combination with bendamustine and rituximab for the treatment of relapsed/refractory diffuse large B-cell lymphoma (DLBCL)” final project plan now available.

The final project plan of the assessment on polatuzumab is now available. The final assessment report will be published on 13th February, 2020. Please access the final project plan at the following link: PTJA06 Final Project Plan
OTCA17 – Final Assessment Report and related documents now available

The final assessment report and related documents for the Other Technologies Collaborative Assessment ‘Lithium triborate (LBO) laser for photoselective vaporisation of the prostate (PVP) in the treatment of benign prostatic hyperplasia (BPH)’ are now available for access. The present assessment addressed the research question whether the lithium triborate (LBO) laser for photoselective vaporisation of the […]
PTJA08 – “Siponimod for the treatment of adult patients with secondary progressive multiple sclerosis (SPMS) with active disease evidenced by relapses or imaging features of inflammatory activity” project plan now available.

The Final Project Plan of the assessment on siponimod is now available. The final assessment report will be published on 13th February, 2020. Please access the Final Project Plan at the following link: PTJA08 Final Project Plan
OCTA20 Final Assessment Report is now available.
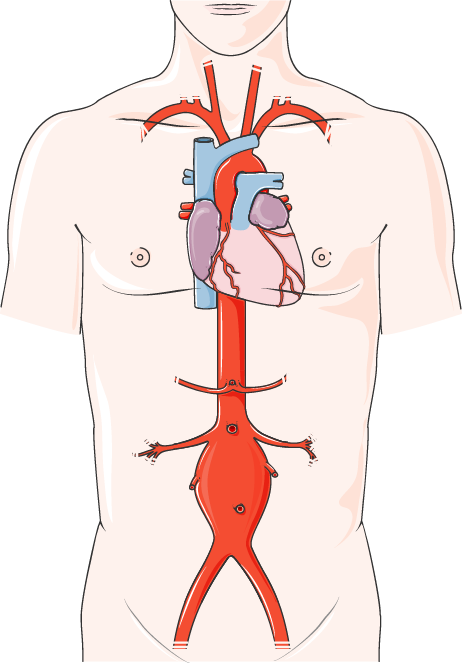
We are pleased to announce that the final assessment report for OTCA20 ‘Prophylactic or therapeutic use of endoanchoring systems in endovascular aortic aneurysm repair (EVAR/TEVAR)’, together with the fact check comments from manufacturers and comments from external experts including the replies of the author, are now available for access. The health technology assessed is a […]
OCTA20 Final Assessment Report is now available.

We are pleased to announce that the final assessment report for OTCA20 ‘Prophylactic or therapeutic use of endoanchoring systems in endovascular aortic aneurysm repair (EVAR/TEVAR)’, together with the fact check comments from manufacturers and comments from external experts including the replies of the author, are now available for access. The health technology assessed is a […]

